Describe the Process Scientists Use to Produce Emission Spectra
Describe how the pigments found on thylakoid membranes are organized into photosystems and how they relate to photon light energy. The specific emission bands of the.
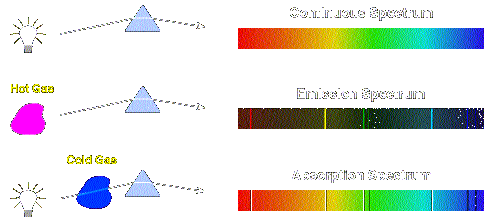
Atomic Absorption And Emission Spectra
The width of the ZPL is determined by the lifetime of the excited state Sauer et al 2010 though in practice it will be broadened by.
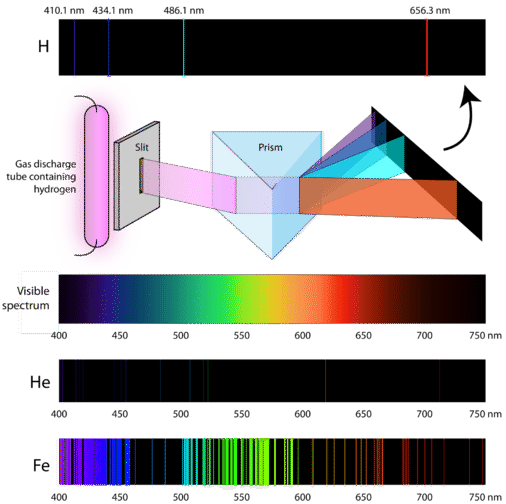
. Photosynthesis is the process by which plants some bacteria. The UCNPs showed up-conversion emission peaks at 345 and 361 nm under the excitation of 980-nm light. Describe the nature of light and how it is associated with the release of electrons from a photosystem.
Figure 2D shows the absorption spectrum of LAP blue line and up-conversion luminescence emission spectra of UCNPs purple line and email protected nanoinitiators black line in an aqueous solution upon 980-nm excitation. Describe the role that chlorophylls and the other pigments found in. However its underlying mechanism may not be understood by scientists who use PL spectra daily.
The ZPL is a sharp peak often referred to in PL spectra precisely because its wavelength is exact specific to a certain defect and unambiguous.
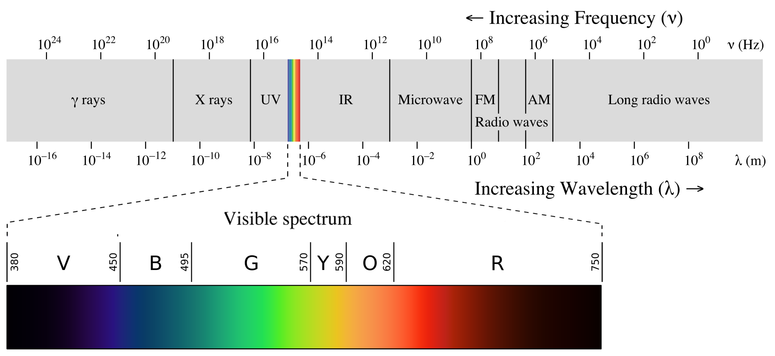
4 2 Understanding Atomic Spectra Chemistry Libretexts
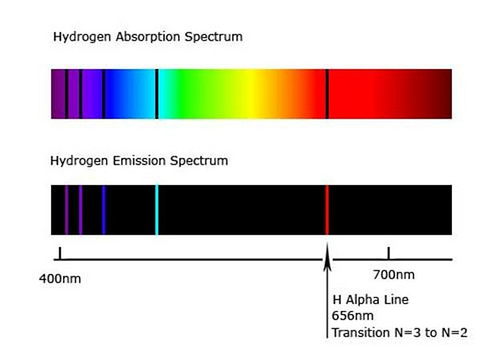
Absorption Spectrum Emission Spectrum Lines Article Khan Academy
0 Response to "Describe the Process Scientists Use to Produce Emission Spectra"
Post a Comment